Law of Conservation of Mass
What is this about exactly???
This section covers the Law of Conservation of Mass.
We also go over how mass is conserved when undergoing physical and chemical changes!
Vocabulary you NEED to know:
The Law of Conservation of Mass: The main principle of physics that states that matter can be neither created nor destroyed.
Matter: the substance or substances of which any physical object is composed of.
Mass: a coherent, typically large body of matter with no definite shape.
Big Idea
Matter is all around us and it has certain properties that allow it to be what it is!
The Law of Conservation of Mass is used to state that matter can be neither created nor destroyed.
Mass is conserved when undergoing physical or chemical change even though the object or piece may be in a different form.
For example if you have Sulfur and Iron, you could add heat and get Iron Sulfide.
The chemicals might undergo a chemical change but will still keep the amount of mass they had together before they had a reaction.
Mass is conserved under any physical or chemical change.
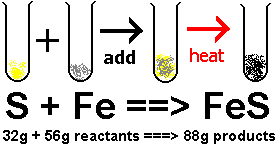

A
B
There are 4 blue molecules and 12 white molecules in Image A.
Now in image B there are the same exact amount of molecules in that picture, but they are rearranged.
If you were to put them both on a scale, they would have the same amount of mass.
Your Turn
What kind of change is occuring in this picture?


EXAMPLES
The pizza still has the same amount of mass even if is cut into slices.

The fruit and the blended up fruit are still the same amount of mass.
